The Science Behind the Sparkle: How Firecrackers Create Light, Sound, and Color
Ever stared in wonder at a fireworks display? There's amazing chemistry and physics at work! Uncover the secrets of how firecrackers produce brilliant light, thunderous sound, and vibrant colors.
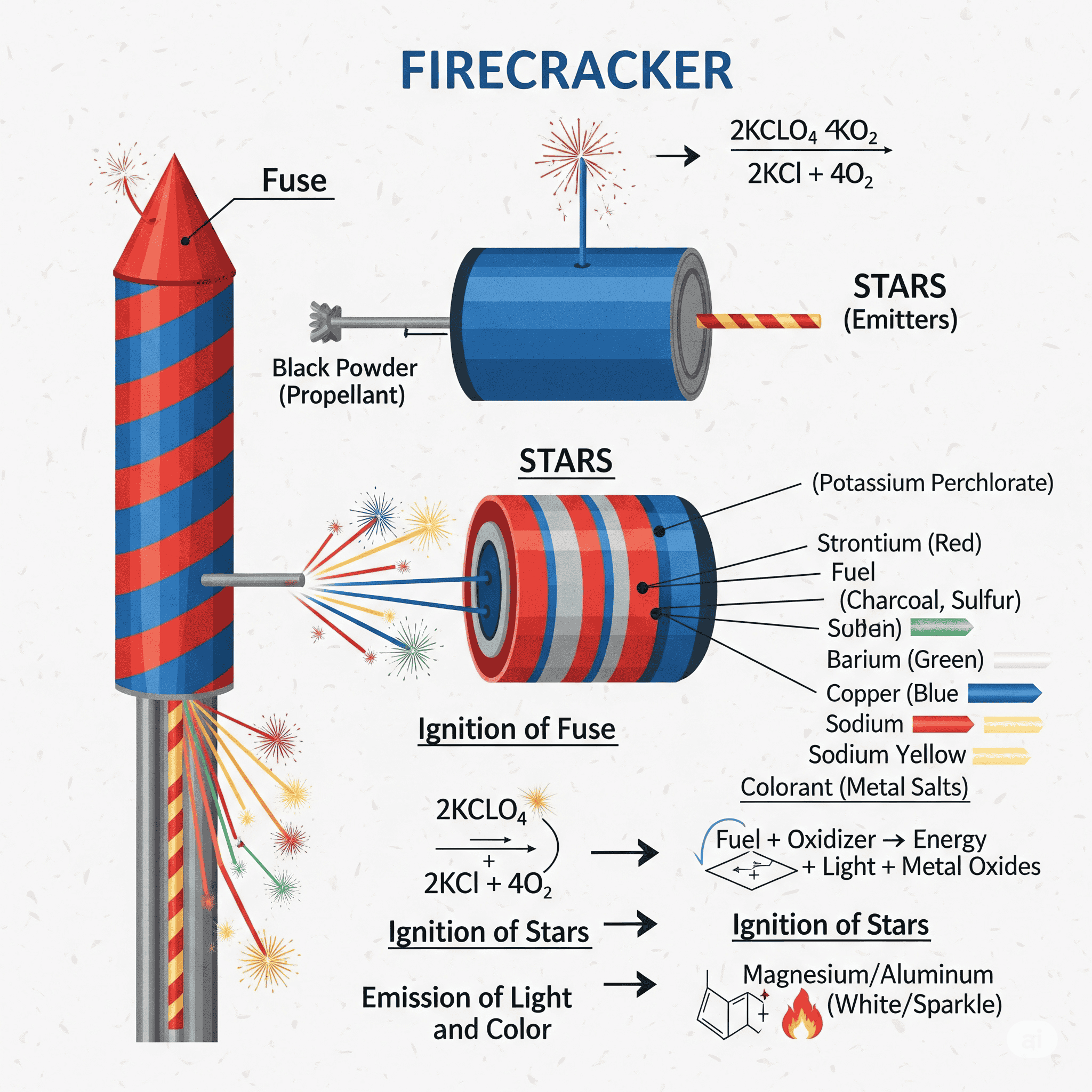
The Basic Building Blocks: Fuel, Oxidizer, and Binder
At its heart, a firecracker is a simple device. The core mixture, called pyrotechnic composition, typically contains three key ingredients:
Fuel: This is what burns. Common fuels include charcoal, sulfur, or metals like aluminum or magnesium.
Oxidizer: This provides the oxygen needed for the fuel to burn in the airless environment inside a cracker. Potassium nitrate (saltpeter) is a classic example.
Binder: This holds the mixture together into a paste that can be shaped. Often, a simple starch or sugar solution is used.
When you light the fuse, it delivers a flame to this mixture. The fuel and oxidizer react in a rapid chemical combustion, releasing a tremendous amount of energy in the form of heat, light, and gas.
Creating the Sound: The Big Bang
That satisfying bang isn't just burning; it's a sonic boom. The combustion reaction happens incredibly fast, producing a huge amount of hot gas. This gas expands violently in the tiny, confined space of the cracker's paper shell. The pressure builds until it’s too much for the shell to contain—resulting in a miniature explosion that rips through the air, creating a shockwave we hear as a loud bang.
Creating the Light: Intense Heat and Incandescence
The brilliant light we see is created primarily through incandescence. The chemical reaction produces intense heat, heating up the solid particles in the mixture until they become white-hot and glow brilliantly. This is the same principle that makes the filament in an old lightbulb glow.
Creating the Colors: The Magic of Metal Salts
This is where the real artistry comes in. To create those stunning reds, greens, and blues, pyrotechnicians add specific metal salts to the mixture. Each element emits a unique, characteristic color when it is heated to a very high temperature, a process known as atomic emission.
Strontium salts produce brilliant Reds.
Barium salts create bright Greens.
Copper compounds yield vibrant Blues.
Sodium compounds give off a warm Yellow/Orange glow.
Calcium salts can create Orange hues.
Titanium or Aluminum powders create dazzling white Sparks and flashes.
By carefully crafting the mixture with these salts, manufacturers can create the mesmerizing multicolored effects we love.
Shaping the Display: Physics in Action
The shape of the cracker and the arrangement of the internal compartments determine the pattern in the sky. Aerial shells are like mini-projectiles. A lift charge propels them into the sky, and a time fuse ensures the main burst happens at the right altitude. Spherical shells create symmetric circles, while cylindrical shells can create willows or ring patterns.
So, the next time you light a sparkler or watch a rocket soar, remember you're witnessing a brilliant application of chemistry and physics.
Ready to see the science in action? Explore our vast collection of brilliantly designed crackers on our website and light up your Diwali with the magic of science!
